
Important breakthrough | jinfeng laboratory team discovered a new subpopulation of tumor-associated macrophages in malignant glioma and its therapeutic significance in normalizing tumor blood vessels
tumor-associated macrophages (tams) highly infiltrate within malignant solid tumors, promoting tumor evolution and the formation of an immunosuppressive microenvironment. at present, the development of tam-targeted immunotherapy strategies is in full swing, including blocking tam recruitment, enhancing tam phagocytosis, regulating tam polarization and metabolic reprogramming, and chimeric antigen receptor-macrophage (chimeric antigen receptor-macrophage) therapy. the number of clinical trials in china is increasing year by year, but its therapeutic effect and translational value are still relatively limited. the reason is related to the high heterogeneity of tam source, intratumoral distribution and phenotype. in-depth elucidation of tam heterogeneity and formation mechanisms is the key to developing new strategies for tam-targeted tumor immunotherapy.
malignant glioma is a primary malignant tumor of the central nervous system that resists conventional treatment, has a high recurrence rate, and has a short survival period. tams in malignant gliomas are formed by the polarization of peripheral monocytes and brain tissue-resident microglia. unlike microglia-derived tams (mg-tams), which are enriched next to tumors, monocyte-derived tams (mo-tams) are mostly enriched in glioma tumors. collection, and its increased content is associated with tumor recurrence and poor patient prognosis. however, whether there is phenotypic heterogeneity in mo-tam in different spatial regions within glioma and its formation mechanism remain unclear.
big discovery
Identification of hypoxic macrophages in glioblastoma with therapeutic potential for vasculature normalization
on april 18, 2024, academician bian xiuwu, associate professor shi yu, and professor ping yifang of the institute of clinical pathology of the first affiliated hospital of army medical university (chongqing southwest hospital) (part-time pi of jinfeng laboratory chongqing institute of advanced pathology) the team collaborated with associate professor qi shuhong from the glioma medical research center of the first affiliated hospital of army medical university, neurosurgery associate professor li fei, and professor zhang zhihong from wuhan optoelectronics national research center of huazhong university of science and technology in cancer a research paper titled identification of hypoxic macrophages in glioblastoma with therapeutic potential for vasculature normalization was published in cell.systematically revealed the heterogeneous characteristics of transcriptional phenotype and spatial distribution of mo-tam in malignant glioma, identified a hypoxic subpopulation of tam (hypoxia-tam) enriched in the hypoxic and necrotic microenvironment, and confirmed that it induces microvascular permeability leakage phenotype, elucidating the therapeutic implications of targeting this subpopulation for normalizing glioma vasculature and improving antitumor drug delivery efficiency.

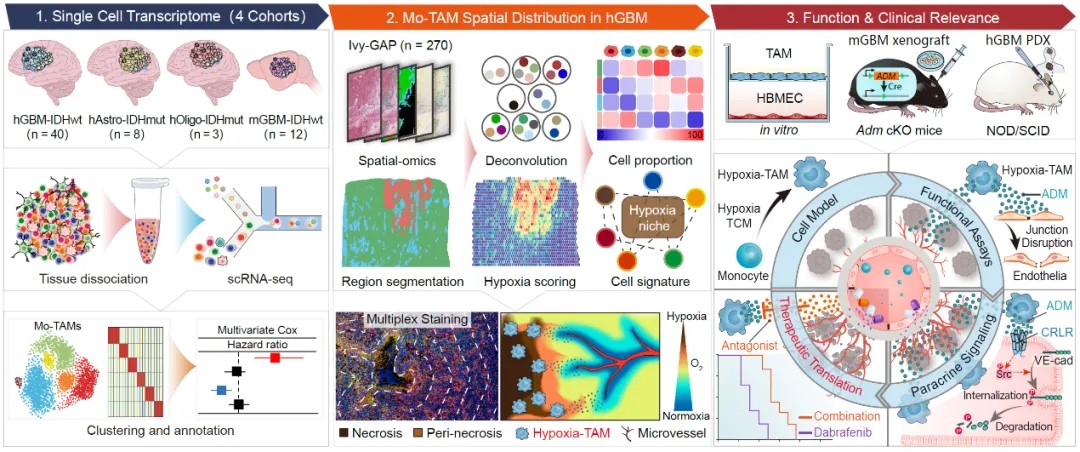
the study includes the following key findings:
1. single-cell multi-omics technology was used to draw the mo-tam spatial map of malignant glioma, and it was found that hypoxic and necrotic areas are highly enriched in hypoxia-tam subpopulations.
the team analyzed 360,000 single-cell transcriptome data in 51 cases of human malignant glioma and drew a single-cell map of mo-tam; identifying cells with hypoxic response, chemotactic motility, phagocytosis and antigen presentation, and lipid metabolism. dependence, interferon production, and ribosome synthesis characteristics of mo - new functional subgroups of tam, and confirmed that the transcriptional characteristics of multiple subgroups of mo-tam are highly conserved in mouse gliomas and various human solid tumors; clarified the relationship between the composition of mo-tam subgroups and glioma types , correlation between tumor grade, tumor molecular variation and patient prognosis. they also analyzed the spatial transcriptome data of 35 cases of human malignant glioma and established the relationship between the transcriptome characteristics of different mo-tam subgroups and the pathological structure of glioma tissue (vascular enrichment area, hypoxic necrosis area and invasion front area). the spatial mapping network drew the spatial distribution map of mo-tam and clarified the distribution differences of different mo-tam subpopulations in the blood vessel enrichment area, hypoxic necrosis area and invasion front area. they used multicolor immunofluorescence imaging to confirm that the hypoxia-tam subpopulation is highly enriched in the hypoxic and necrotic areas of gliomas, and analyzed the spatial hypoxia gradient and hypoxia-tam phenotypic transformation and glioma cell-mesenchymal transformation. , correlation between glycolytic metabolic reprogramming and microvascular proliferation.
2. elucidate the key mechanism by which hypoxic and necrotic microenvironment induces phenotypic polarization of hypoxia-tam subpopulations
the team used human peripheral blood monocytes and mouse bone marrow-derived macrophage models in vitro to analyze the role of tumor factors and hypoxic factors in the hypoxic and necrotic area of malignant glioma in inducing the polarization of monocytes toward the hypoxia-tam phenotype. influence. through integrated multi-omics analysis of bulk transcriptome, proteome, and glycolytic metabolites, we systematically screened and identified cysteine-rich acidic secreted proteins produced by glioma cells in the hypoxic necrotic microenvironment. acidic and rich in cysteine, sparc) and hypoxia-induced lactate are key mechanisms for the polarization of hypoxia-tam subpopulations, confirming that the two factors synergistically induce p50 in mo-tam to enter the nucleus and enhance the transcription of hypoxia-tam core phenotypic genes.
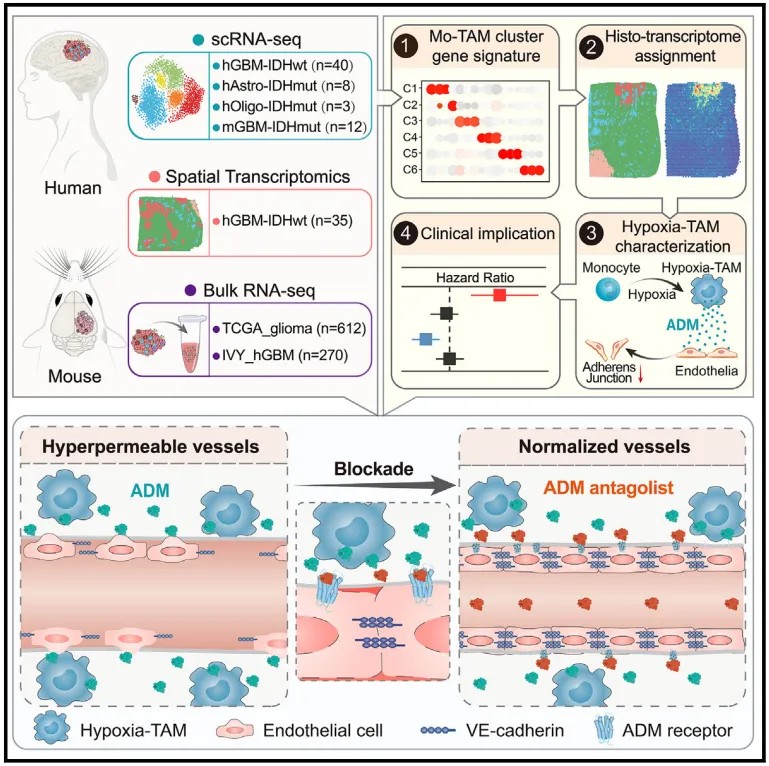
3. it was found that hypoxia-tam is an important reason for inducing high leakage of microvessels in malignant gliomas, and a new treatment strategy was established to target hypoxia-tam to induce normalization of glioma blood vessels and improve the delivery and efficacy of anti-tumor therapeutic drugs.
highly proliferated, hyperleakage, and low-perfusion glioma microvessels are important factors that limit antitumor drug delivery and lead to tumor cell drug resistance. in view of the large number of newly formed microvessels surrounding the hypoxic and necrotic lesions of malignant gliomas, the team further analyzed the effect of hypoxia-tam on angiogenesis and the structural integrity of microvessels, and found that hypoxia-tam can synthesize and secrete adrenomedullin in large amounts ( adrenomedullin (adm), leads to the destruction of adherent junctions between microvascular endothelial cells in gliomas, thereby causing vascular hyperleakage and blood flow hypoperfusion. knocking out adm in monocytes and their derived cells in ccr2-cre transgenic mice, or using adm antagonists to block the adm paracrine pathway, can inhibit angiogenesis in mouse gliomas, restore the integrity of endothelial connections and reduce blood vessels leakage, thereby inducing normalization of intratumoral microvascular structure. they confirmed in braf-v600e mutated human glioblastoma xenografts that the combination of an adm antagonist and the braf small molecule inhibitor dabrafenib can increase the intratumoral perfusion of dabrafenib and be more effective. inhibit tumor growth and prolong survival of tumor-bearing mice.
in summary
this study used single-cell multi-omics technology to map the tam spatial map of malignant glioma, identified new functional subpopulations of hypoxia-tam in the hypoxic microenvironment and elucidated their formation mechanism, and proposed and established a targeted antagonism of hypoxia-tam paracrine adm is a new therapeutic strategy to induce the normalization of glioma blood vessels and improve the perfusion and efficacy of anti-tumor therapeutic drugs. the above content has deepened our understanding of the spatial heterogeneity characteristics and formation mechanism of the immune microenvironment of glioma, and is expected to be used to promote new immunotherapy strategies and new anti-tumor angiogenesis strategies targeting tams from different sources and functions in glioma. strategy development.
master's student wang wenying, doctoral students li tianran and cheng yue from the department of pathology (institute of pathology), southwest hospital of army medical university, associate professor li fei from the department of neurosurgery, and associate researcher qi shuhong from the wuhan national research center for optoelectronics at huazhong university of science and technology are the joint first authors of the paper. . academician bian xiuwu of the department of pathology (institute of pathology) of southwest hospital affiliated to army medical university, associate professor shi yu, and professor heping yifang (part-time pi of jinfeng laboratory chongqing institute of advanced pathology) are the co-corresponding authors. professor feng hua and professor hu rong of the department of neurosurgery, the first affiliated hospital of army medical university, professor liu xindong (part-time pi of jinfeng laboratory), professor wang yan (part-time pi of jinfeng laboratory), and dr. liu haofei (jinfeng laboratory) of the institute of clinical pathology feng laboratory researcher), professor zhang zhihong of wuhan national research center for optoelectronics of huazhong university of science and technology, professor zhu bo of the second affiliated hospital of army medical university (part-time pi of jinfeng laboratory), etc. also provided suggestions or technical guidance for this research.
- About Us
-
Research Platform
- Major Disease Sample Database
- Innovative Drug Verification And Transformation Platform
- Experimental Animal Center
- Life And Health Future Laboratory
- Biomedical Imaging Platform
- Cell Multi-Omics Platform
- Pathology Technology Platform
- Bioinformatics Research And Application Center
- Jinfeng Pathology Precision Diagnosis Center
- Research Team
- Information Center
- Join Us


 渝公网安备50009802002274
渝公网安备50009802002274