
The scientific research team of jinfeng laboratory jointly discovered that neutralizing il-8 can improve the therapeutic effect of immune checkpoint blockade in brain glioma
"on march 23, 2023, liu xindong, bian xiuwu, wang yan's team from the first affiliated hospital of army medical university and jinfeng laboratory, and lu shengqing's team from the second affiliated hospital published an online paper on cancer cell entitled neutralizing il-8 potentiates immune checkpoint blockade efficacy for glioma the article reveals the role of il-8 in constructing the immunosuppressive microenvironment of brain glioma and its potential application value as an immunotherapy target, providing new insights into the immunotherapy of brain glioma. "

the functional status of tumor-infiltrating t cells is a key factor in determining anti-tumor immune responses and immunotherapy responses. in most cases, more tumor-infiltrating t cells indicate a stronger anti-tumor immune response. however, the content of t cells in the glioma microenvironment increases with tumor grade, suggesting the functional diversity of glioma t cells. the latest research shows that clonally expanded tumor-specific t cells in brain glioma mainly include three subpopulations: exhaustion, effector, and memory [3]. however, the functions of the remaining non-clonally expanded t cells in tumors are still unclear. . researchers in this article first analyzed the distribution of t cells in brain glioma through histology and found that t cells were mainly distributed around blood vessels. analysis based on the ivy gap database also proved this conclusion. in order to further explore the chemokine receptors that mediate the migration of t cells into tumor tissues, the authors used mass spectrometry flow cytometry (cytof) to comparatively analyze the expression of chemokine receptors in the peripheral blood and tumor tissues of glioma patients and found that tumor-infiltrating t cells highly express the chemokine receptors ccr5 and cxcr3, suggesting that these two receptors are involved in mediating the migration of t cells into tumors.
in order to systematically analyze the functional status of t cells in the tumor microenvironment, the authors performed single-cell sequencing and comparative analysis of t cells in the blood and tumors of glioma patients, and found that tumor-infiltrating t cells included 18 subpopulations, including 7 cd4+ t cell subsets, 10 cd8 t cell subsets and one γδ t cell subset. notably, the authors discovered a subpopulation (th8) that highly expressed il-8 in tumor cd4+ t cells. the low level of th8 tcr clones indicates that they are non-tumor-specific t cells, which highly express il8, il1b, ccl3 and other secreted factors as well as the transcription factor bhlhe41, and are negatively correlated with patient survival. given that th8 is only present in tumor tissue, the authors speculate that factors secreted by tumor cells induce the formation of th8. to test this conjecture, the authors used the culture supernatant of primary brain glioma tumor cells to induce naive cd4+ t cells from healthy people. they found that the tumor cell culture supernatant could induce t cells to release il-8, and the t cells induced by the tumor supernatant the cells have similar transcriptional profile characteristics to th8 cells in vivo and exhibit the characteristics of innate immune cells. through in vivo and in vitro experiments, the authors demonstrated that t cells induced by tumor supernatant have the ability to recruit myeloid suppressor cells (mdsc) and promote blood vessel formation. in order to further explore the intrinsic regulatory mechanism of th8 cell differentiation, the authors overexpressed a series of transcription factors in t cells. the results showed that overexpression of bhlhe41 can strongly induce t cells to express il-8. subsequent functional experiments showed that overexpression of bhlhe41 t cells have th8-like characteristics. however, when the authors used crispr-cas9 to knock down bhlhe41, no downregulation of il-8 was found, indicating that bhlhe41 is a sufficient but not necessary condition for cd4+ t cells to express il-8.
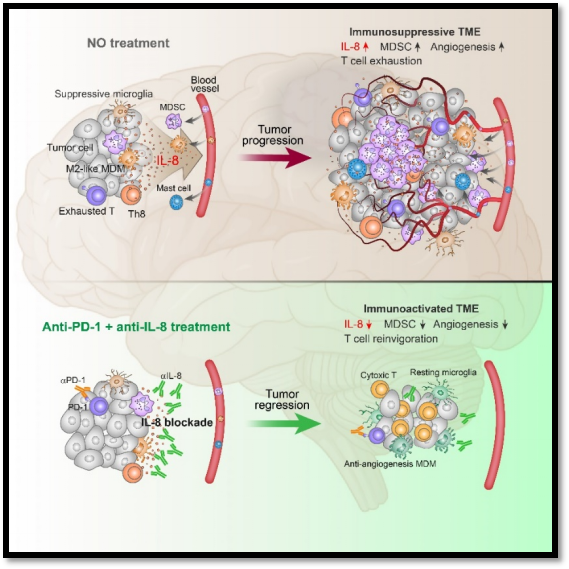
clinically, serum il-8 levels are positively correlated with patient tumor burden and negatively correlated with icb treatment effect [4,5]. however, since the il8 gene does not exist in the rodent genome, the immunomodulatory function of il-8 in tumorigenesis remains unclear. to explore the immunoregulatory function of il-8 in vivo, the authors constructed il-8 fully humanized mice (il8-hu). gliomas in il8-hu mice grew faster, contained more mdscs in the tumor tissue, and had similar histopathological characteristics to clinical samples. anti-pd-1 monotherapy failed to inhibit tumor growth in il8-hu mice. further studies found that pd-1 blockade treatment increased il-8 levels in tumor tissue and serum, leading to increased mdsc infiltration in tumor tissue. this phenomenon drove the authors to conduct combined il-8 and pd-1 blockade therapy. in line with expectations, the combined treatment of il-8 and pd-1 almost blocked the recruitment of mdscs to tumors, inhibited tumor angiogenesis, reduced t cell exhaustion, and significantly prolonged the survival of mice.
in order to more comprehensively explore the impact of il-8 blocking therapy on the tumor immune microenvironment. the authors performed single-cell sequencing analysis on mice treated with different treatments. consistent with previous findings, mdscs in tumors were significantly reduced after blocking il-8. through cluster analysis of monocyte-derived macrophages (mdm) and microglia, the authors found that m2 macrophages and immunosuppressive microglia were significantly reduced in tumors after blocking il-8, while anti-vascular increased mdm generation. this shows that blocking il-8 changes the differentiation trajectories of macrophages and microglia and reverses the tumor immunosuppressive microenvironment.
in summary, this article uses multi-omics research methods to reveal the distribution characteristics and functional characteristics of t cells in glioma. a population of cd4+ t cells (th8) that highly express il-8 was discovered and characterized. th8 cells work with tumor cells and myeloid cells to build a tumor immunosuppressive microenvironment by releasing il-8. blocking il-8 can reverse the immunosuppressive microenvironment of tumors and enhance the therapeutic effect of immune checkpoint blockade (figure 1).
professors liu xindong, academician bian xiuwu, and wang yan from the first affiliated hospital of army medical university and jinfeng laboratory, and professor lu shengqing from the second affiliated hospital of army medical university are the co-corresponding authors of the paper. dr. liu haofei from the first affiliated hospital of army medical university and jinfeng laboratory is the first author.
references
1 Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422-442 (2018). https://doi.org:10.1038/s41571-018-0003-5
2 Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309-5337 (2021). https://doi.org:10.1016/j.cell.2021.09.020
3 Mathewson, N. D. et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 184, 1281-+ (2021). https://doi.org:10.1016/j.cell.2021.01.022
4 Yuen, K. C. et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 26, 693-698 (2020). https://doi.org:10.1038/s41591-020-0860-1
5 Schalper, K. A. et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nature Medicine 26, 688-+ (2020). https://doi.org:10.1038/s41591-020-0856-x
- About Us
-
Research Platform
- Major Disease Sample Database
- Innovative Drug Verification And Transformation Platform
- Experimental Animal Center
- Life And Health Future Laboratory
- Biomedical Imaging Platform
- Cell Multi-Omics Platform
- Pathology Technology Platform
- Bioinformatics Research And Application Center
- Jinfeng Pathology Precision Diagnosis Center
- Research Team
- Information Center
- Join Us


 渝公网安备50009802002274
渝公网安备50009802002274